A Primer of the Iran War, Pt 4.
Israel and Iran: Finale and Coda
Today we finish with the Israeli-Iranian Air War. In Israel it’s come to be called the Twelve Day War, and I wish we could have finished these posts in twelve days. Also I apologize for both the length of this final post—We have too much to cover!—and the omissions—There’s so much I’ve left out!
Last go around we reached the late 1980s. The Islamic Republic of Iran had defeated their secular neighbor, Iraq, after many years of brutal war and intense sacrifice. Most Iranians believed they had overcome the world, since the West and most of the Arab states had backed Iraq. In 1989 the first “supreme leader” of revolutionary Iran died and his successor Ali Khameni took over.
Meanwhile Israel pulled out of Lebanon in 1985 when it became too costly to keep troops there.1 In a sense this was the first war Israel had ever lost; their “Vietnam.” But worse, because it wasn’t just an ego blow; the war birthed a new Shi’ia militant group called Hezbollah, inspired by revolutionary Iran’s defeat of Iraq. Hezbollah turned out to be a far tougher enemy than the secular PLO.
In 1992, Hassan Nasrallah became Hezbollah’s secretary-general. He had fought in the war and spent time afterwards studying in Iran. Under his brilliant leadership Hezbollah increased ties to Iran, particularly importing rockets and missiles and learning to use them effectively. Also Hezbollah renounced pure Islamist separatism to join Lebanese secular politics. In the postwar peace when all militias were supposed to disarm, Hezbollah claimed it couldn’t because the last pro-Israeli militia called the Southern Lebanon Army (SLA) wouldn’t—but Hezbollah tried to make up for it in Lebanese public opinion by forming alliances with other Lebanese groups, offering charity and social services to the ravaged country, and successfully running candidates for the national legislature.
Note that Shi’ia have been persecuted by Sunnis for centuries, and Lebanese Shi’ia by Lebanese Christian militias much more recently, yet Shi’ia Hezbollah gladly made peace with and provided social services to both. Even with Israel, which Hezbollah explicitly proclaimed to be illegitimate and blamed for Lebanon’s Palestinian refugee crisis, Hezbollah made no actual demands beyond that Israel completely withdraw from Lebanese territory.
Giving Up Is Hard To Do
But Israeli governments couldn’t quite bring themselves to do that. They insisted out of pride on maintaining a “security belt”—a strip along the Lebanese side of the border. (If it was really about security they could just as easily have put the security belt on their own side of the border.)
This encroachment ensured both permanent insult and periodic cross-border incidents which became the pretext for war in 1993, again in 1996, and yet again in 2000.
In every case after some incident Israel would unleash massive airstrikes against Lebanon while Hezbollah would fire rockets into Israel. Since Israeli governments promised their constituents that the world was full of dangerous enemies but that the IDF would protect them,2 Israeli government felt obligated to evacuate entire towns at every flurry of rockets. Then they’d revenge these insults with another round of brutal airstrikes, inevitably killing scores of civilians and destroying civilian infrastructure. Then Hezbollah would fire back a few more rockets. BOOM BOOM BOOM. Spst spst. BOOM BOOM BOOM. Spst spst. Back and forth it went. Eventually the outside world, horrified by the destruction in Lebanon, would negotiate a peace.
Israeli governments hoped their airstrikes would discredit Hezbollah, strengthen the pro-Israeli SLA, and cause enough civilian deaths and misery to force the Lebanese government to do what Israeli couldn’t do—disarm Hezbollah. Surely Lebanon would learn the price of tolerating terrorism. Surely the Lebanese would be rational enough to see that Israel meant business. After all, Israel was inflicting far more damage than it was receiving—it was “winning”—so the Lebanese should see reality.
But as we’ve said before military conflicts are about military objectives not deaths and destruction, and Israel lost these conflicts as soon as they started bombing. Airstrikes may have made Israeli politicians look strong domestically, but they made Israel look feckless and cruel internationally. They caused the Lebanese to turn against the SLA as the only Israeli proxy they could reach. And as for expecting the Lebanese government to somehow disarm Hezbollah, if Israel couldn’t disarm Hezbollah, then how could the weak Lebanese government disarm Hezbollah? By essentially telling Beruit, Choose us or them, Israel forced Lebanese governments to forge closer ties with Hezbollah.
Mostly, Israeli complaints about the inhumanity of Hezbollah rockets looked ridiculous in light of far higher casualties and destruction Israel was inflicting on Lebanese civilians. If the world is supposed to be outraged by a Hezbollah rocket injuring one person in some Northern Israeli town, how can the world not notice that Israel just killed twenty in a flattened apartment complex in Beirut? Israel made it seem like Israel—and Israel’s supporters—fully expected the world to care more about Israeli Jewish lives than Lebanese Arab lives.
Israel bombing Lebanon—so militarily pointless, vindictive, cruel—became the story in the Muslim world not just of contemporary Israel but retroactively of the original 1982 invasion of Lebanon, and by extension the entire Israeli state. There had always been people looking for any reason to hate Israel, and always people (not always the same people) who sympathized with Palestinian refugees, but eighteen years of bombing Lebanon made any anti-Israeli argument seem reasonable to Middle Eastern ears.
Strategically it was a disaster too. By 2000 when Israel finally agreed to withdraw to the “blue line”—the original UN resolution of 1978—the SLA was gone and Hezbollah was in undisputed control of the Southern third of Lebanon, the defenders of the country in the eyes of a plurality of Lebanese, and the heroes of the Muslim world.3
Yet even in 2000 for no strategic reason Israel insisted on continuing to occupy a 7x2 mile speck of useless land called Shebaa Farms, claiming bizarrely that it was actually Syrian territory which they were illegally squatting on so they didn’t have to give it back to Lebanon. Syria claimed otherwise, but of course Israel didn’t care what Syria actually thought.
Life is Unfair
Lebanon may be Israel’s Vietnam, but the effects aren’t the same. Countries like France, Great Britain, and the United States just have it easier than Poland, Germany, and Russia. France has navigable rivers, a mild climate, seacoasts and mountains. 5/6 of the border isn’t accessible to an invading army. England once it united with Scotland has no open border at all. The United States is a vast continent protected by oceans with no real enemies.
Poland, Germany, and Russia, meanwhile, are literally surrounded by potential enemies who can invade from many directions; they have tough climate issues; tough navigation issues, and so on.
Likewise throughout history most of the powerhouses in the Middle East have arisen from Anatolia (Modern Turkey), the Iranian plateau (as we have seen), or occasionally Egypt. Those are relatively defensible positions from which empires can expand or retreat. States based in these territories can muddle around, screw up, and still have second, third, fourth, eightieth chances.
Not so for countries like Lebanon, Syria, Israel, Jordan, and Iraq—or their ancient counterparts. Their mistakes can cost them their literal existence. We saw in Part I the ancient Kingdom of Israel picked the wrong side in one war and was obliterated forever. The ancient Kingdom of Judah picked the wrong side in one war and would have been obliterated too if not for the timely defeat of their Babylonian oppressors.
And lest you think this precarity comes from international hatred of Jews or Jewishness, note that non-Jewish states in the same vicinity haven’t had it easier. We’re not hearing much about Ammon, Moab, the Cannanites, Philistines, or the Phoenicians these days. The medieval Kingdom of Jerusalem and other “Crusader” states are long gone too, and they had as much outside military and financial support as Israel today. And of course contemporary Lebanon, Syria, and Jordan face a challenge even Israel has never faced—sharing a border with Israel.
For some countries mistakes are moral; for countries like Israel, Lebanon, Syria, and Jordan, mistakes can be existential. It isn’t world opinion that holds Israel to a higher standard than Egypt, Turkey, or Iran; it’s demography and geography.

Sunnis, Israelis, and Iraqis, Oh My.
Speaking of countries and their Vietnams, the U.S.S.R. inherited all the challenges of its Russian heartland, but learned few of Russia’s lessons—nor any of ours. Despite the obvious blowback from America’s 1958 coup in Iran, the U.S.S.R. engineered a coup in neighboring Afghanistan in 1973 to bring a Marxist party to power there. That party soon split between moderates and radicals. The U.S.S.R. favored the moderates believing the radicals would push too hard and incite a national uprising. But the radicals won out, and sure enough, they pushed too hard and incited a national uprising. The desperate government then appealed for help from the Soviet Union, and the Soviets, like a dad bailing a foolish son out of jail while shaking his head I-told-you-so, felt duty-bound to send in the troops.
Thus began the Afghan War, the Soviet Union’s Vietnam.
The U.S. immediately recognized it could be the U.S.S.R.’s Vietnam, and poured money and weapons in through Pakistan to guarantee it. It worked! In 1989, with the Red Army demoralized and depleted and the Soviet empire wounded4 the U.S.S.R. gave up and withdrew. The Afghan government fell. The country was left a dystopian hellscape of broken lives, souls, and bodies that the Taliban eventually took over, but the U.S. government was on to other challenges, pausing only for a few chest bumps and high fives of victory.
I mentioned in an earlier post that during this period the U.S. was encouraging Saudi Arabia to found Sunni religious schools all over the Middle East. These schools were Islamist (as opposed to merely Islamic), meaning they envisioned Islamic law as the basis for government. They also wanted to strip Islam of corruptions and heresies and so on, but Islamic law was a direct challenge to the official atheism of the Soviet bloc, so that’s why U.S. Big Thinkers thought it was smart to support.
The big three Islamist approaches, the Muslim Brotherhood, Salafi Islamism, and Wahhabism,5 all influenced Khomeini and revolutionary Iran, but those Sunni Islamists viewed the Shi’ia—even successful Shi’ia revolutionaries—as heretical so there wasn’t much cultural exchange, which meant the U.S. Big Thinkers may have imagined in their zero-sum way that helping the Sunni Islamists might weaken Iran.
In any case the Big Thinkers in Israel had the same bright idea and were funding a minor Islamist off-shoot of the Muslim Brotherhood called Hamas to weaken the secular PLO.6
I don’t know much of the success of Islamist movements came from support by the intelligence agencies, how much from their own passion and organizational strength, and how much from the weakness of other approaches, but it’s important to note that as the Ottoman and later British and French Empires withdrew from the region the people of the Middle East overwhelmingly turned first to secular movements like the Ba’athist parties of Syria and Iraq, Arab Nationalism and Pan-Nationalism, and secular Palestinian Liberation Organization, and only when the monarchs and secular movements failed to deliver did the Islamist movements really take fire.
People need to know the rules to function in life, and in the midst of chaos many will crave the rule of law. Law that everyone must follow, that everyone has a right to know, that is consistently applied. Now Islamic Law is a dreadful body of law in my opinion, but it is definitely a body of law, and for many that may have seemed better than the arbitrary rule of cops, security services, and governments on the take.7
Anyway during the Afghan War the U.S. and Pakistan discovered that the Sunni Islamist networks—so dedicated, organized, and secretive—were perfect for funneling weapons and money to the Afghan rebels. So America and Pakistan encouraged those pipelines and relationships and helped train Sunni jihadists all over the Middle East to develop their organizational skills and social connections. What could go wrong? One minor fundraising and training group was founded by Pakistani Sunni Islamist Abdullah Yusuf Azzam and his wealthy Saudi Arabian Sunni Islamist construction-company-owning protege named Osama Bin Laden.
It’s Sunni Time!
Now we must leave Iran, Hezbollah, and the Shi’ia to follow up more with these Sunnis because they impact Israeli-Iranian relations too. We’ll go through it quickly, and pick up with Iranian-Israeli relations at the far end.
1990. Iraq, crippled by debt from its long war with Iran, can’t convince its creditors to give it slack. Iraq considers this ungrateful since wasn’t it fighting Iran for everyone? Much of that debt is owed to Kuwait, and Kuwait is particularly unhelpful about accepting another I.O.U., so Iraq invades Kuwait. Bush Sr responds with the Persian Gulf War. (Eventually shortened to the “Gulf War,” so that those dang Iranians aren’t part of the name.)
1990-1992. During the Gulf War at the Saudi monarchy’s request the U.S. stations troops in Saudi Arabia. Osama Bin Laden, home after the Soviet withdrawal from Afghanistan, is outraged and begins to criticize the monarchy. He’s expelled to Sudan where he heavily invests in business and construction with much of the same crew Pakistan and indirectly the U.S. had helped him organize in Afghanistan.
1994. Israeli-American extremist and West Bank settler, Baruch Goldstein kills 29 worshipers and wounds 125 at a mosque in the West Bank, leading to widespread Palestinian protests during which Israeli police kill 25 more Palestinians and puts Palestinians (but not Israeli settlers) under curfew.
1995. Another Muslim Brotherhood offshoot in Gaza—a rival of Hamas—Palestinian Islamic Jihad (founded in Gazan refugee camps8) begins to carry out suicide bombings against Israelis, particularly on buses.
1996. Regretting their early support of Hamas, Shin Bet (Israeli intelligence) assassinate Hamas military leader in January. Hamas carries out retaliatory public suicide bombings (competing with rival PIJ perhaps?) that discredit the government and help to elect outsider Bibi Netanyahu as Prime Minister. Hamas continues to carry out terrorist attacks throughout Netanyahu’s term in office, eventually leading to his defeat in his reelection bid.
1996. Driven out of Sudan by pressure from Saudi Arabia and the U.S., Bin Laden and his crew move to Afghanistan, and declare themselves at war with the U.S. for the crimes of stationing troops in Saudi Arabia, for the suffering in Iraq caused by the Gulf War, and for supporting Israel. Over the following years this organization comes to be called Al Qaeda. They bomb three U.S. embassies in East Africa. In retaliation President Clinton fires cruise missiles at sites in Sudan and Afghanistan. More terrorist attacks follow over the years, some foiled, some not.
2000. Ariel Sharon elected prime minister of Israel. He builds the separation walls and checkpoints in the West Bank, and in 2005 withdraws from Gaza without negotiating with Hamas or Fatah over how it would be governed. Gaza collapses into anarchy and Hamas is able to seize control. By this time Hamas has seen the success of Hezbollah’s rocket strategy in periodic fights with Israel and begins to construct and fire their own homemade rockets.
2001. 9/11. Osama bin Laden later says the reason he approved the plan for flying planes into the Twin Towers with all the expected civilian deaths was because of American support for Israeli during its wars with Lebanon. He wanted Americans to know what it felt like to be murdered from the skies.
2001. The U.S. provides air support and special forces to aid rebel Afghans to overthrow the Taliban government of Afghanistan. The Taliban flees to Pakistan. Osama Bin Laden flees as well and goes into hiding under protection of elements of Pakistani intelligence or military.
2002. Between political offices Bibi Netanyahu gives “expert” testimony before U.S. Congress strongly advocating for the U.S. invasion of Iraq. He guarantees the invasion will bring "enormous positive reverberations" across the region.
2003. America invades Iraq. American neoconservatives emboldened by the quick defeat of the Taliban (or so they assumed) put together a fraudulent case for the war based on “Weapons of Mass Destruction,” forged accounts of uranium smuggling, and fake ties between Iraq and Al Qaeda.
2006. Remember Shebaa Farms? Hezbollah captures some soldiers there as bargaining chips to get captured Lebanese released from Israeli prisons. Israel responds with a yet another pounding of Lebanese civilians and infrastructure and tries to invade. Hezbollah fires missiles back into Israel. After a month Israel withdraws.
2007-2008. In September of 2007 Israel initiates total blockade of Gaza in revenge for rocket attacks. After four months of diplomatic efforts fail Hamas retaliates with vastly increased rocket attacks. Israel responds with Operation Cast Lead, a carbon copy of those periodic Lebanese poundings. Airstrikes cause immense destruction and civilian death. Eventually Egypt brokers a deal where Hamas will prevent rocket attacks while Israel will lift the blockade. Hamas keeps its side of the bargain for three years, but Israel never lifts its blockade. (It hasn’t to this day.) The Israeli government promises to keep Gaza’s economy "on the brink of collapse."
All this may look bad in summary, but trust me, it’s even worse if you dig into it all.
Bibi Time
Sometime between Prime Minister Sharon building the separation wall and Prime Minister Ehud Olmert’s total blockade of Gaza, Israel politics died.9 Before this period Israeli political culture had been dynamic, with different parties taking power, and real debate and the genuine possibilities for new directions and outcomes. There was media from across the political spectrum, including genuine pro-Palestinian voices, and Israeli historians were doing incredible work. Every Israeli prime minister (Including first-term Netanyahu) at least considered peace initiatives. And while all governments lie, it always seemed like Israeli governments at least knew when they were lying. The bad stuff was always there too, but it was only part of the picture.
But in 2009 Bibi Netanyahu was elected prime minister for a second term. Whether you consider Netanyahu the cause, result, or merely symptom of Israeli stagnation, he’s been prime minister ever since. There is no serious domestic opposition any longer, his policies are popular even when he isn’t, and he seems to get his way at every turn. Today Netanyahu is Israel as surely as Putin is Russia. Maybe not tomorrow, certainly not forever, but today that’s the reality.
How did that happen? Domestically, any left-leaning governing coalition has to include the Labor Party—which has long represented secular Zionist Jews—but the Labor Party was unwilling to put Palestinian Israelis in positions of power in a cabinet. That worked okay for Labor when they were super popular and successful, but when they lost popularity—as all parties will—they just didn’t have the votes without Palestinians.
So just as the U.S. Democratic party by refusing to embrace “far left” policies like taxing the rich, breaking up monopolies, or providing Americans with healthcare basically cedes election advantages to the Republicans, so the Labor Party’s refusal to work with Palestinians cedes most Israeli elections to right-wing coalitions.
Right-wing coalitions depend on far-right parties including crazy religious settlers. It’s popular in American circles to lament those religious fanatics, but remember, if center-left Jewish Israelis had included Israeli Palestinians in real decision-making capacities, then the religious crazies would have never been in a government.
By Netanyahu’s second term, if not before, all Israeli governments have treated law-abiding, cooperating Palestinians as badly as Palestinians in actual terrorist groups, and Israeli government foreign policy consists of assassinations, propaganda, airstrikes, assassinations, propaganda, airstrikes in mindless rotation.10
#1 Sponsor of International Terrorism Seeks to Wipe Israel off the Map!
Which brings us finally to the nukes!
There was a time from the 1950 into the 1970s when nuclear power was the world’s shining symbol of progress. In 1953 President Eisenhower got the ball rolling with his Atoms for Peace speech to the fledgling United Nations,11 proposing the creation of what became the International Atomic Energy Agency (IAEA) to observe, advise, and regulate nuclear programs around the world for safe and peaceful uses. In 1950s UN diplomats began working on a Nuclear Non-Proliferation Treaty (NPT) that was first signed in 1968 (by Finland!) and over the decades all the countries of the world except for India, Israel, Pakistan, and South Sudan.12
So back in the 1950s naturally the Shah of Iran wanted nuclear power to prove what progress he was bringing to Iran. He was a prized U.S. lackey at the time, so the U.S. said, Why not! By the 1970s Iran had signed the NPT, joined the IAEA, and reactors were under construction, but the revolution stopped all that. Khomeini saw nuclear power as a symbol of the decadent West, and besides, the war with Iraq absorbed all money and resources.
In the 1990s with the war over and Khomeini gone, Khamenei decided to pick up where the Shah left off, this time with new friends. Russia (The U.S.S.R. had collapsed.) began to help Iran build reactors and China helped it created domestic uranium mining and processing. The IAEA was invited in for inspections.
As I understand the physics (and better educated people, please correct me in the comments) real live uranium, or any element when it’s mined, doesn’t always have the number of protons, neutrons, and electrons listed on that high school table of the elements that hung over Mr Heepe’s desk. In the mix are always other atoms called isotopes that have a different number of neutrons. Nuclear reactors need a certain of those isotopes. The isotopes are separated from the standard elements—enriched as its called—by putting uranium in a machine called a centrifuge that spins. I picture the rock tumbler my brother kept running in our bathroom when we were kids.13
To build an atomic bomb is apparently not too hard. (I couldn’t do it; I couldn’t even build my brother’s rock tumbler.) The reason there aren’t more atomic bombs in the world is that it’s expensive and tough to get the fissile material. For a bomb the uranium (or plutonium or whatever) needs to be spun far longer, i.e. enriched more, than for a reactor.
More than that, atomic bombs aren’t very useful. Except for the United States at the end of WWII no country has figured out what to do with them, but countries develop them because other countries have them. The Soviet Union developed nukes because the U.S. had nukes; China developed nukes because the Soviet Union had nukes; India developed nukes because China had nukes; Pakistan developed nukes because India had nukes. It was Pakistan who helped North Korea develop its nukes.14
No one can actual use these without other countries potentially retaliating. However, nuclear weapons do seem to get countries treated better. Certainly, the U.S. and Israel treat Pakistan a lot better than they treat Iran. Many in United States and Israel probably assume that Iran must be trying to get nuclear weapons because if they were in Iran’s shoes that’s what they would do.
Of course none of us really know, but in 2005 IAEA inspections found Iran in violation of safety regulations and reporting obligations, and Iran agreed to suspend uranium enrichment to clean up shop. Khamenei’s government published his fatwa against production, storage, and use of nuclear weapons or any “weapons of mass destruction”—a decree that allegedly had been given in house two years before—a new inspection regimen was worked out, and in 2006 Iran resumed uranium enrichment completely within international law.15
During that year a traffic engineer named Mahmoud Ahmadinejad was elected president of Iran. Of course Iranian presidents are not actual heads of state—Khamenei was in charge—but in the midst of the nuclear scandal Ahmadinejad was quoted by the international press as quoting an earlier statement by the late Supreme Leader Ayatollah Khomeini that was (mis)translated into English:
Israel must be wiped off the map.
That phrase is still cited today in virtually every pro-Israeli diatribe against Iran. I don’t speak Farsi, and I’m betting you don’t speak Farsi, but the two Farsi speakers that I’ve known both told me—as innumerable Farsi speakers have written online and elsewhere—that that actual quote was more like, The thing will fade from the pages of history. Or something like that. “The thing” was Israel, of course, which you might consider threatening enough, but what Khomeini definitely did not say, and what no Iranian leader has ever said, was the Iran would cause Israel to disappear, that Iran would attack Israel, or that Iran had any duty to do anything about Israel. Khomeini was in fact reassuring the Iranian people that Iran didn’t need to be scared of Israel or fight Israel, since it would disintegrate by the hand of Allah soon enough.
Khamenei, Khomeini, and most of the Islamists view Israel—"The Zionist entity” or “The Zionist regime”—as the West dumping the problems caused by Europe’s Holocaust, Europe’s antisemitism, on the Middle East. Rather than returning the survivors of the Holocaust to their rightful homes in Europe or carving out a state for the survivors in Europe, they stole land from the Muslims. Islamists imagine the Muslim world—at least when properly ruled by Islamic Law—as having never being antisemitic. No true Scotsman, true Muslim ever persecuted Jews or anyone else, because the Prophet said not to! This last claim is ridiculous on many levels, but about the Palestinians paying the price for the crimes of the Nazis, there’s truth in that, as most early Israeli leaders acknowledged.
But Ahmadinejad was far more than an anti-Zionist with a sanitized view of Muslim history. He loved media attention and he loved saying provocative things about the country he refused to call Israel. At every opportunity he claimed it was illegitimate, cancerous, always about to be eliminated. Ahmadinejad embraced true Western-style antisemitic conspiracy theories too. He spent time while president organizing a “conference” for Holocaust deniers and skeptics. (So in his thinking the Europeans or Jews or Zionists hate Muslims so much that they basically faked the entire Holocaust in order to have an excuse to humiliate Muslims by creating Israel.)
The Iranian Jewish community complained about Ahmadinejad’s idiocy, Khamenei criticized him, but Netanyahu got a lot of mileage out of claiming Iran was obviously crazy and therefore willing to destroy itself to harm Israel.16
Again and again Khamenei reissued his fatwa asserting that Iran and no Muslim nation could use nuclear weapons, and said again and again that Iran would never attack Israel. Again and again, historians and scholars pointed out that Israel had nukes of its own and no country—certainly not Iran—would destroy itself just to harm Israel, but it made no difference. The Iranians can’t be trusted. The Iranians are just evil.
Israel began assassinating numerous Iranian (civilian) nuclear scientists, and Israel and/or the U.S. created malware called Stuxnet which sabotaged Iranian computers at one nuclear facility in 2009. (And spread around the world infecting other computers I’m told but I don’t know about that.)
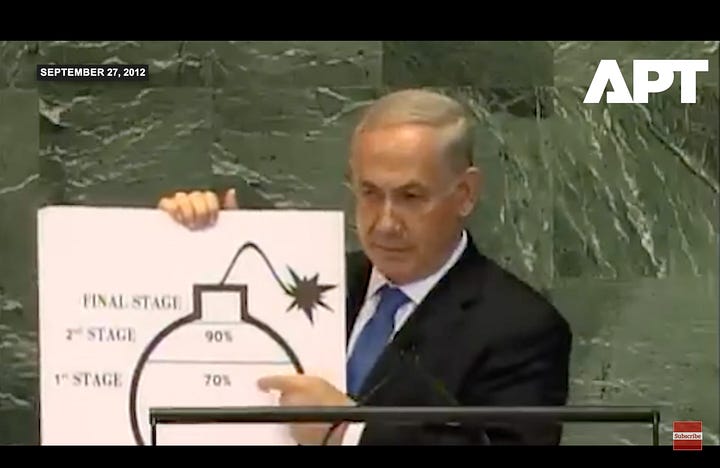
Peak Netanyahu was achieved in 2012 when the man gave a speech at the United Nations telling the world Iran was only months away from nuclear weapons while holding a drawing of a Wile E Coyote cartoon bomb.
At this point if you don’t understand why assassinating scientists, infecting computers, and giving speeches while holding cartoon bombs is not going to deter countries from whatever they’re doing, I don’t know what to tell you. Maybe Netanyahu is so sure that Iran was trying to develop an atomic bomb because that’s what he would do. If Iran wasn’t bomb-making before—and all the evidence is they weren’t—sooner or later Netanyahu probably figured they would start. More probably he was just angry at Iran’s support for militant groups on Israel’s border, but we’ll get to that.
To satisfy Netanyahu in 2015 Obama negotiated the Joint Comprehensive Plan of Action (JCPOA) which paired short-term freeze on enrichment with easing of sanctions and greater access for inspections. Once again Iran promised not to enrich uranium to a weapons-grade level, and now there were clear-cut means of checking this. It was a good treaty and by all accounts followed by all parties, even though Netanyahu was so NOT satisfied he unsuccessfully lobbied against it and against Obama’s reelection.
Later when Trump come to office, despite no record of violations, the U.S. government unilaterally disavowed the treaty and resumed the sanctions. As in all Trump actions one suspects he was sucking up to donors or just proving what a tough guy he is. Still Iran continued to abide by its part of the deal until 2019 when Trump assassinated an Iranian general on a diplomatic visit to Iraq. Iraq was ostensibly our ally so this was an act of war against both Iraq and Iran.17
In response Iran formally announced it no longer considered the 2015 treaty binding, but continued to invite the IAEA in to monitor their facilities. When further desperate efforts to negotiate the removal of the sanctions got no traction with Trump or Biden, Iran formally announced they would enrich past the level needed for most domestic uses, but stop well short of what was needed for a bomb. The IAEA would have full access for inspections to verify this stance.
This was supposed to pressure the U.S. to reconsider sanctions. Instead, well, you’ll see…
The Rockets Red Glare
Meanwhile in Yemen a brutal Shi’ia Islamist insurgency called the Houthi had been fighting a long, brutal guerilla war against the brutal Sunni Islamist government. In 2015 the Houthi took control and the Sunnis appealed to Saudi Arabia who organized a long, brutal blockade and even more brutal aerial war against what now was the only government of Yemen. A horrible famine ensued and the Saudi campaign has been credibly accused of being genocidal, a sort of trial run for Israel in Gaza. But no one noticed because it was Yemen.


The Houthi rallied under a banner called the Sarkha whose rabid antisemitism makes Ahmadinejad sound like a reasonable guys in comparison:
God is the Greatest
Death to America
Death to Israel
Curse be upon the Jews
Victory to Islam
Iran has disavowed nuclear weapons and attacking Israel, but they’re never disavowed funding and supporting militant groups, especially Shi’ia militant groups. Iran supported (Shi’ia) Hezbollah early and often with money and weapons, and the (Shi’ia) Houthis by the time they were in power.
When we get to the Netanyahu period everything is so much stupidity and propaganda that it’s hard to get accurate information. At various points Iran began also supporting two Sunni militant groups that were enemies of Israel. Both Palestinian Islamic Jihad and later Hamas were supplied with money, technical assistance, and in Hamas’ case eventually rockets, though never the higher-tech missiles Iran gave to Shi’ia militias.18 Anti-Israeli sources present Iranian funding as always reactions to Israeli provocations, i.e. Iranian support for bus bombings in Gaza was a reaction to killings in the West Bank, or actions against Iran, i.e. assassinations of nuclear scientists. Pro-Israeli sources present Iranian funding as motivated by “wiping Israel off the map.” I can’t even piece together an accurate timeline.
But one reason I started this series with a history of Iran itself is because I want to get across that Iranians don’t hate American Great Power Politics because they hate Great Power Politics, but because they hate what America does with its Great Power. The Iranians largely consider the Great Power Politics of their ancestors, the Medes, Persians and Parthians, as being forces of good in the world. They do not see themselves as some sort of small, backward country; they see themselves as a regional power under relentless attack by Israel and the United States. They are not crazy jihadists hoping to die for Allah, nor are they cowards intimidated by Israel or America; they are rational actors—far more rational these days that contemporary Americans and Israelis.
The Air War
On Oct 7th, 2023 Hamas sent 6000 militants slipping across the border of Gaza into Israel in more than a hundred locations. There had been ample warnings but Netanyahu either ignored them because he was caught up in legal problems or he assumed it would be a small incursion that would give him an excuse for retaliatory “mowing the lawn” to shore up his popularity.
The militants kidnapped more than 250 hostages. The Israeli government quickly or immediately opted for massive collective punishment against the people of Gaza, and this choice has been very popular with Jewish Israelis. The intense and deliberate murder, torture, home destruction, ethnic cleansing, propaganda, and starvation in Gaza will be considered one of the half-dozen most horrifying episodes of mass criminality of the last two centuries.
The conflict expanded into (yet another) invasion of Lebanon, including a successful terrorist attack by Israel that assassinated Nasrallah and forced Hezbollah to accept a ceasefire in Israel’s favor. A war in Syria overthrew its secular Ba’athist dictatorship and brought Sunni Islamist dictatorship to power. Exchanges of rockets and airstrikes with the Houthis have tended to play out in Houthi favor for the Houthi have managed to strangle trade in Israel’s Red Sea port. In the first year of the war a few missile exchanges with Iran had little impact on either.
Trump took office and imposed a ceasefire on Gaza (which Netanyahu quickly broke) and set out to negotiate a new JCPOA-type framework with Iran. For someone as chaotic and fickle as Trump it was an auspicious beginning. Yet while those negotiations were ongoing on June 13th Israel suddenly attacked Iran as we wrote about here. Iran shot missiles back, and Israel and Iran went back and forth, with the U.S. expending a lot of our stockpiles in Israel’s defense.
As we said in the first post of these series Trump expended yet more U.S. resources to strike Iran directly. Iran hit a U.S. military base in response. Now we have a ceasefire, but from a strategic point of view none of this benefits the U.S. and little of it benefits Israel.
The Israel assassinations and bombing involved information taken from the IAEA either through theft, bribery, or corruption, so Iran has withdrawn from IAEA inspections. This is a catastrophe. Now if Iran decides to develop a bomb no one will know it.
Israel has continued its genocide of Gaza, Netanyahu announcing Israel will take control of the entire territory. Gazans will be moved into concentration camps, and those who refuse to go will be subjected to military attack.
Israeli settlers protected by the government have been committing an increasing number of atrocities in the West Bank against Palestinians who aren’t in any way part of Hamas. Members of the cabinet have credibly been accused of planning to ethnically cleanse and annex the entire West Bank once Gaza is finished.
Israel is working with the Lebanese government to try and disarm Hezbollah and ethnically cleanse the Shi’ia from South Lebanon, moving them to Iraq. This will probably lead to another Lebanese Civil War.
The U.S. is arming an area of land leased from Armenia along Iran’s northern border. (Israel’s attack on Iran came from this area.) Trump stationing U.S. troops on the Iranian border. What could go wrong?
The biggest lesson from the Middle East—reiterating the lessons from Ukraine—is that rockets, missiles, and drones are the future of warfare. Every competent military in the world is now stockpiling rockets, missiles, and drones. Missile defenses don’t work and are much too expensive in comparison to missile attacks. Since missiles can be fired from portable locations there’s no military answer.19 Not yet anyway. That means tanks lose their original reason for existing and aircraft carriers are as obsolete as battleships were in WWII.
As far as Israel and Iran, I assume Israel will attack Iran again and Trump will probably help out, or attack Iran on his own. If Iran feels the survival of its regime is at stake they’ll answer with their full arsenal and indeed wipe Israel off the map, as this particular Iranian regime will likely be wiped off that map in return.
Neither in any case will be erased from the pages of time. Many Persias have arisen from the Iranian plateau and if this one is destroyed others will follow incorporating whatever from the current Iran survives. I hope Iran doesn’t succeed in its revolutionary goal of spreading Islamic Law, but on that issue my Magic Eightball only tells me, Hazy Try Again.
As for Israel, Iran and the Houthis can’t stop the Israelis from killing a million Gazans, and no one else is going to do it either. A few countries are talking about “recognizing a Palestinian state” as an answer to domestic public pressures but that’s months away, by which time Israel will have starved, shot, and bombed tens of thousands more, and what does Netanyahu care about foreign countries “recognizing a Palestinian state”?
There is no hope for the people of Gaza—a million will starve to death while the world does nothing—but that strip of land is not the hills of Rwanda or the rice fields of Cambodia; it’s on the Mediterranean coast with innumerable journalists and foreign medical workers and aid workers, and whistleblowers, and videos.
The stories will come out for decade after decade of the displaced, the tortured, the crippled, the traumatized, from the families of the dead and their descendants, from the guilt-ridden Israelis and their enablers. Every bulldozer and backhoe removing debris will unearth more corpses, mangled, starved, raped. As the Nazi Holocaust continued to impact American life for generations,20 the destruction of Gaza will change American politics, Israel, Zionism, and Judaism too. Within twenty years the Western meritocracy who allowed it to happen will be gone, utterly discredited. (Whether we get something worse or better I cannot say.) Within eighty years Israel will be gone. Zionism will be disavowed by most Jews long before that.
The whole painful process won’t even begin until the shooting stops, and the shooting won’t stop until Netanyahu is gone.
In summary Truman was right and Eisenhower and the CIA were wrong; it would have been better just to let Mossadegh have those oil wells.
Thanks for reading Blame Cannon! For the next couple of months I’ll circle between a series on global warming in prehistory, a story about coal, and a series of true stories about departures and goodbyes.
Please subscribe and share!
Comments are welcome but please no personal insults or profanity.
*The Abc News Story from the picture.
If it wasn’t clear in the previous post, this was officially Israel’s second invasion of Lebanon, the first being a briefer 1978 affair in which Israel invaded to push PLO forces out of the south. The UN pressured Israel to withdraw, so Israel withdrew and the PLO returned.
“Israeli Defense Force.” The Israeli army.
Despite the SLA being gone Hezbollah still never quite got around to disarming. Now they claimed they couldn’t disarm because of Israel.
How much the Afghan War mattered in fall of the U.S.S.R. is a subject of debate.
The Muslim Brotherhood was founded in Egypt, Wahhabism was founded in Saudi Arabia, and Salafism is roughly Wahhabism for non-Saudis.
Hamas is an acronym for Islamic Resistance Movement that puns on the Arabic word for “bravery.” The Israelis were funding it around 1991. The usual story is that they did this to weaken the PLO, but it might be they were trying to co-opt a rival to the Palestinian Islamic Jihad.
That may well have been like the Maya figuring Cortez couldn’t be worse than the Aztecs. I very much doubt that Islamic Law in practice is ever arbitrary and cruel than secular systems, but the grass is always greener. By the way, this drift from secular approaches to religious approaches in the Middle East isn’t just the Muslim world; Israel has followed that same path.
Most of the people of Gaza are refugees from ethnic cleansing of Israel during Israel’s Wars of Independence. PIJ was founded by Islamists in those camps. The Al-Quds Brigade is part of the PIJ.
As ours did sometime between the Iraq War and Trump’s first election. Sharon, Olmert, and Begin (who invaded Lebanon in 1982) were all of Israel’s right-wing Likud party. So is Netanyahu.
The Israeli concept of propaganda, “hasbara” means “explanation” and includes both public relations and lobbying.
This was only the eighth session of the U.N.
North Korea signed the treaty as a condition to get assistance from the Soviet Union but was kicked out for non-compliance. I don’t know anything about South Sudan.
Well, it’s probably nothing like that.
North Korea never really wanted nukes. It threatened nukes to get bribes from the west which most U.S. presidents were happy to offer. Then genius Mr Deals President Donald Trump in his first term refused to give North Korea bribes because he was too tough for that, and North Korea called his bluff and built nuclear weapons.
Khamenei pointed out that Iran even in the face of Iraq’s use of chemical weapons had never responded with chemical weapons.
Of course there’s an irony in Netanyahu and his cabinet complaining about Ahmadinejad’s idiocy, given that they—including Bibi “Amalek” Netanyahu himself—say equally belligerent and crazy things quite often.
Iran retaliated by bombing a U.S. military base.
Hamas’ first rockets were homemade, but once they proved themselves, Iran gave them or sold them better rockets, thought not the sophisticated equipment Iran has given to Hezbollah and the Houthis.
In Ukraine drones have been able to knock out tanks pretty effectively, so those don’t work either. However, I don’t know how much drone and missile communications can be jammed.
The English edition of Diary of Anne Frank was published in 1952. The hit television miniseries, The Holocaust, aired in 1978. 2025’s Oscar-nominated film The Brutalist centered on a survivor of the Nazi Holocaust.


Regarding Uranium Enrichment:
Chemical elements are defined by their Atomic Number, which is the number of protons in the nucleus of each atom. Two atoms with different numbers of protons are by definition different elements. Atoms also include electrons (whose negative charge offsets the positive charge of the protons) and neutrons (which have no charge). Electrons are nearly weightless and live in concentric "shells" around the nucleus. Neutrons weigh the same as protons and, like them, live in the nucleus. The Atomic Weight of an atom is the number of protons and neutrons in the nucleus. Isotopes of an element contain the same number of protons but different numbers of neutrons. For example U-237 has an Atomic Weight of 237.
The reason uranium is radioactive is that it is unstable. It emits particles as part of a long slow process called radioactive decay whereby each atom loses protons and thus turns into a different element. In the case of uranium the element it turns into isn't stable either and it keeps decaying until it turns into lead. We consider lead to be heavy because it is one of the heaviest elements that is stable and relatively common.
The process of decay is slightly different for the different isotopes of uranium. Some isotopes emit considerably more energy over a given time span than others, and this difference is important when creating nuclear fission, which is the sudden splitting of atomic nuclei into two or more smaller nuclei of lighter elements. Fission can cause an explosion or, if contained, a sustainable reaction useful for generating power (as well as various scientific purposes).
The concept of enrichment is kind of a metaphor based on milk. We call cream richer than skim milk because it contains more fat. Because it contains more fat it is lighter and naturally rises to the top, where it can be skimmed off and separated from the "whole" milk. We call certain isotopes richer because they produce more radiation. Because they have different numbers of neutrons per atom, different isotopes have different Atomic Weights and can be separated by weight just like the components of milk - under the right conditions.
I'm not sure how they make uranium, a solid metal, into something that acts like a liquid, but I do know that unlike milk it does not spontaneously separate by weight under normal gravity. In order to promote this separation they use a centrifuge, which is simply a wheel that holds the uranium and spins it very quickly. The material in the centrifuge wants to move in a straight line, and would do so if the wheel fell apart, but instead is continually redirected into a curved path. The force that the uranium exerts against the wheel in resisting this change of direction is called centrifugal force, and in many ways it acts like gravity, causing the uranium to separate into isotopes like milk separating into cream and skim milk. In this way, after many rounds of spinning and skimming, a more "rich" blend of isotopes can be extracted from the naturally occurring mix.